Executive Summary
What if the body could be programmed to make its own medicines? It is a question that has been posed by scientists, clinicians and public- and private-sector players for years. Little did anyone know that, as the Covid-19 virus spread and a global pandemic took hold, the world would find out.
Pfizer-BioNTech was not the first vaccine to prove effective against Covid-19 but it was the first to use mRNA technology. The significance of this cannot be overstated. Not only was it a key part of the global fightback against the disease (without it, there would certainly have been more deaths and economic disruption than there was) but, crucially, it also proved that the technology worked and could be deployed at pace and scale, paving the way for further research.
Since then, the advantages of mRNA as a treatment modality have started to become clear. First is the potential breadth of applications it has as a platform technology, meaning it can be used as the basis for developing a range of diagnostics, therapeutics and care-delivery solutions. For example, it can be deployed against Covid-19, viruses and infectious agents but also cancer, autoimmune conditions, and genetic and neurodegenerative conditions. Some of these applications are in the early phases of research and development; others, like cancer therapeutics, are already being tested to treat patients in clinical trials.
A second key advantage of mRNA is the difference it could make to the speed and cost of manufacturing treatments, because the treatments generated by platform technologies share common pathways in design and build. This means a potentially speedier path to drug discovery, lower costs of production (because newer drugs would have some of the same base manufacturing processes as older ones) and greater agility in responding to biosecurity and pandemic threats.
The third advantage to highlight is the potential for treatments to be more effective. Treatments can be tailored to the individual by combining mRNA technology with genomic medicine; this improves their effectiveness and reduces the incidence of side effects, heralding a new era of personalised medicine.
It is clear that platform technologies will have a profound impact on patients, health systems and economies – and mRNA will be one of the most transformational. The country that cracks the development of mRNA technologies will be well placed to lead the world in other platform technologies, as it will have the skills, infrastructure and blueprint for rolling out new personalised treatments. It would result in a kind of mRNA multiplier effect.
The United Kingdom should be at the forefront of this new frontier in medical science. It has all the ingredients it needs: world-class academic research institutions, a strong industry presence from biotech and pharma, an internationally respected regulatory framework through the Medicines and Healthcare products Regulatory Agency, and a National Health Service that works closely with academia and ivndustry to research and develop new treatments.
But to cement its place as a world leader in mRNA technology and attract inward direct investment, the UK needs to present a clear end-to-end pathway for the design, manufacture, testing, regulation, procurement and delivery of these medicines to the world. To be part of this mRNA revolution – and a leader in the future of personalised medicine – the UK also needs to make a strategic decision to invest. The United States, Australia and the European Union are already doing so, which means the competition is intense. Plus, the UK has gaps.
-
Capacity for clinical trials: Trials activity in the UK has fallen dramatically in recent years, as life-sciences companies look to other countries with a more attractive trials environment.
-
Capacity for mRNA manufacturing: There is also limited manufacturing capacity. mRNA requires specific facilities that meet good manufacturing practice mRNA-LNP specifications, of which the UK has only limited supply.
-
Regulation of mRNA technologies: Platform technologies present unique challenges to the traditional approach to drug regulation – and the UK’s current approach is fragmented and confusing.
-
NHS procurement mechanisms: The NHS has rigid procurement mechanisms that protect it from high costs, but which also limit the UK’s ability to attract inward investment from R&D companies.
-
Infrastructure: The NHS is not yet set up to deliver personalised medicine at scale; this would require changes to its physical, logistical and data infrastructure.
-
Skills: The UK lacks both clinical and technical expertise across the piece – from research development and manufacturing to regulation, procurement and delivery of personalised medicine.
Beyond investment, the UK also needs strategy and coordination. Having the component parts of the pathway is not enough: it is the seamless transition from one end to the other that will determine effectiveness. During the pandemic, these factors came together through the expert shepherding of the Vaccines Taskforce. It was this group that pulled together those disparate component parts in a purposeful, coordinated way, and led to the UK delivering the first ever mRNA Covid-19 vaccine. Now, it is the role of two government departments – the Department of Science, Innovation & Technology and the Department of Health & Social Care – to work together in the same way, spurring collaboration between life-sciences companies and the NHS to develop new innovations and drive economic growth in the UK.
Finally, and most importantly, what the UK needs to show is leadership. Some of the gaps identified in its end-to-end pathway are easy fixes but others require ingenuity – and it is here that the UK can really shine. Imagine the UK pioneering an entirely new approach to the regulation of personalised medicines, setting the ball rolling in other countries and kickstarting a revolution in health care that makes personalised treatments accessible to all (a kind of regulatory diplomacy). Already this year we have seen BioNTech’s has committed to delivering cancer immunotherapies to 10,000 patients by 2030,[_] and Moderna’s 10-year investment in an Innovation and Technology Centre with planned capacity to produce up to 250 million vaccines.[_] Industry players have confidence in the UK as a world leader in biotech – now the UK must back itself to succeed.
The Case for Investing in mRNA Technology in the UK
Platform technologies will have a profound impact on patients, health systems and economies. The country that cracks mRNA technologies will be well placed to lead the world in other platform technologies, as it will have the skills, infrastructure and blueprint for rolling out new personalised treatments – a kind of mRNA multiplier effect.
There are a number of key reasons why mRNA should be considered a transformative technology:
-
Speed: Once the genetic sequence of a target protein (a virus spike protein, for example) is known, mRNA vaccines can be designed and produced rapidly, shortening product-development lifecycles and potentially bringing treatments to patients more quickly.
-
Flexibility and scalability: The same mRNA platform can be developed for different targets by changing the sequence, meaning it can be used to rapidly respond to emerging threats and diseases.
-
Safety: Safety data borne out by the successful application of many other vaccines, such as the Covid-19 vaccine that more than 1 billion people have used, means there is potential to speed up regulation and guidance.
-
Versatility: mRNA has applications in cancer immunotherapy, rare genetic diseases and regenerative medicine, making it a versatile platform on which to build a multitude of different vaccinations and therapeutics.
The UK should be at the forefront of this new frontier in medical science. It has all the ingredients it needs: world-class academic research institutions; a strong industry presence from biotech and pharma; fantastic computational and AI talent; an internationally respected regulatory framework through the Medicines & Healthcare products Regulatory Agency (MHRA); and a National Health Service that works closely with academia and industry to research and develop new treatments.
The development of the Covid-19 vaccine demonstrated the benefits of mRNA as a platform technology, malleable enough to build vaccines to prevent illness from infectious disease and ultimately saving millions of lives. This platform technology also promises a new era of personalised medicine, with treatments that can be precisely tailored to individual genetic profiles, dramatically improving therapeutic effectiveness while minimising side effects. Building out the UK’s mRNA capabilities will benefit patients, the NHS and the economy if the right measures are taken.
For patients, mRNA offers hope across a spectrum of challenging medical conditions. Beyond its proven success in vaccine development, the technology holds potential breakthrough applications in cancer treatment, autoimmune disorders, genetic conditions and neurodegenerative diseases. Each of these represents a frontier where traditional treatments have fallen short of delivering for patients.
For the NHS, mRNA technology presents a strategic advantage: its platform approach enables faster, more cost-effective drug development, with shared manufacturing processes that could significantly reduce production timelines and costs. This efficiency could transform the NHS’s ability to respond to emerging health threats, creating a more agile, responsive and secure health-care system.
From an economic perspective, mRNA technology represents a golden opportunity for national leadership in medical innovation. By leveraging its world-class research institutions, robust biotech and pharmaceutical sectors, internationally respected regulatory frameworks, and integrated health-care-research ecosystem, the UK is uniquely positioned to become a global leader in this transformative medical technology, which would attract the likes of researchers and private-sector companies to base themselves in the country.
The potential is not just incremental but multiplicative. Success in mRNA could establish the blueprint and infrastructure for future platform technologies, creating high-value scientific and manufacturing jobs, attracting international investment, and positioning the UK at the forefront of a global medical-technology revolution.
In essence, mRNA is more than a technological advancement: it is a strategic national opportunity to redefine health care, drive economic growth and improve patient outcomes across multiple domains. It is up to the government to make some strategic decisions and invest in key areas across regulation, procurement, infrastructure and skills to turn this potential into reality.
What Is mRNA?
The acronym stands for “messenger ribonucleic acid” and is effectively the communication material between the cells’ DNA “blueprints” and their building blocks, or proteins. When a cell decides it needs to produce more protein – for example a signalling protein to activate immune cells – it opens up the relevant DNA blueprint, finds the correct instructions for the manufacture of that protein within the genetic code and copies theses instructions into mRNA form. This process is known as “transcription”.
These transcribed mRNA molecules are similar to structural drawings that an architect would produce for builders. The mRNA then shuttles from the cell’s nucleus, where the DNA is stored, into the outer part of the cell called the cytoplasm. Here the mRNA is “translated” by specialist structures called ribosomes – the ribosomes “read” the mRNA, just like a builder interpreting plans, and construct the protein.
This biology explains why mRNA is so versatile: it is a set of written instructions that tell bodies’ cells to produce any protein of interest. It is possible to make mRNA that can instruct the manufacture of any protein – but common ones are viral or cancer proteins, in the case of infectious disease or cancer vaccines, or immune proteins to manipulate the immune system. While mRNA biology has long been studied, it is manufacturing advances in the past decade or so that have improved its stability and delivery to cells within the body. That is what has made this breakthrough concept a medical reality.
The term “platform technology” is commonly used in reference to mRNA. Medical platform technologies are foundational scientific or technological advancements that serve as versatile enablers for developing a diverse range of diagnostics, therapeutics and care-delivery solutions. These platforms are designed to be broadly applicable across disease areas, allowing researchers, clinicians and companies to leverage core capabilities, tools and frameworks to rapidly build innovative products.
In the case of mRNA platform technology, it is possible to develop a common process for designing, manufacturing and testing novel mRNA therapeutics. So although each “drug” will differ in terms of granular design, indication and intent (meaning the code will instruct the manufacture of different proteins), it will share many common features with other therapeutics across the same platform, whereby simple alteration of the coding sequences can provide the patient’s body with instructions to produce different therapeutics. This makes it easier to predict and validate quality, safety and clinical effect, and could substantially accelerate the process of drug discovery and development.
This is a technology that has a wide range of transformative human applications that are being observed across many disease types, providing the opportunity to improve global health. In infectious-disease vaccines, mRNA can rapidly target viruses including Covid-19, influenza, RSV, HIV and Zika, as well as other pathogens (such as those that cause malaria) by encoding antigens that elicit immune responses.
The personalisation of medicine is also possible with mRNA. For example, cancer is currently thought of by organ type – breast, prostate, colon – but two colon cancers may behave in completely different ways. Personalised medical treatment would mean examining features of that individual cancer and selecting – or even producing – a drug that best fits. The versatility of mRNA makes it an ideal platform for producing truly bespoke treatments, which will lead to improved outcomes for patients while also minimising side effects.
For cancer, personalised and general “off-the-shelf” therapeutics can direct the immune system against tumour proteins, aiding in the treatment of cancers such as melanoma, lung and even hard-to-treat pancreatic cancer. In autoimmune diseases, mRNA enables precise immunomodulation, potentially treating conditions such as type 1 diabetes, multiple sclerosis and lupus by manipulating immune activity.
When it comes to rare diseases, mRNA can support protein replacement (for immunodeficiencies or enzyme deficiencies, for example) and potentially gene editing in inherited diseases such as cystic fibrosis, haemophilia and muscular dystrophy, providing functional proteins or enzymes where needed. In addition, mRNA could be used in cell therapy to improve treatments for cancers and genetic disorders by temporarily programming cells to perform specific tasks, such as attacking tumours or producing missing proteins.
The true value of mRNA comes in the versatility of its applications and scalability, going from population-wide infectious-disease vaccines to personalised cancer immunotherapies. In contrast to the status quo for most medicines – a complex design and manufacturing process that is developed and maintained for one drug at a time – mRNA platforms can be rapidly adapted to generate therapies across disease types and indications. From infectious disease to cancer, from rare genetic disorders to common chronic illnesses, there is a role for mRNA. It has vast potential in terms of health improvements for the UK population, as well as profound economic implications. Currently the UK is a world leader in scientific research in many fields where mRNA has potential, and we urge the government to capitalise and build on this to unlock the significant benefits of mRNA.
The versatility of mRNA technologies means they can be used as both vaccines and therapeutics
Phases of the mRNA Development Cycle That the UK Should Be Investing In
As outlined, there is overwhelming potential for mRNA technology to benefit patients and the economy. However, the end-to-end ecosystem needs some work to get to a point where this potential can be realised, particularly with regards to clinical trials, manufacturing, procurement and delivery infrastructure.
Clinical Trials
The UK’s position as a leading global destination for running mRNA clinical trials is long established. As the pandemic demonstrated, the country has the ability to run large-scale trials at speed using mRNA technology, with medicines reaching patients quickly. Off the back of this success came the added benefit of further investment, with businesses setting up shop here. However, maintaining this position, and the substantial benefits that such status brings, should not be taken for granted.
Clinical trials should be a critical part of how the UK considers its strategy in marrying health and life sciences. It is the closest industry gets to the patients and the NHS, trialling novel therapeutics intended to deliver better care outcomes. The placement of small early-experimental through to large-registration efficacy studies provides opportunities for patients to access novel (and potentially efficacious) treatments free of charge, and far in advance of these therapeutics being licensed for prescription.
Not only do patients get access to cutting-edge treatments quicker, but clinical trials at all phases are also a significant source of income for NHS trusts and the life-sciences sector in the UK. As noted in a previous TBI paper, A New National Purpose: Harnessing Data for Health, the UK has a 3.8 per cent share[_] in a global clinical-trials market currently worth £67.6 billion.[_] Industry clinical trials in 2022 contributed £7.4 billion in gross value added (GVA) to the UK economy and created 65,000 jobs. The NHS directly benefited from £1.2 billion in revenue, with 13,000 jobs within the NHS. Trials helped avoid 3 million sick days, saving £0.9 billion for the UK economy. Returning clinical-trial activity to 2017 levels could add £3 billion – including £486 million for the NHS – and 26,000 more jobs.
Clinical trials represent an opportunity for the NHS to be paid to deliver cutting-edge treatment to patients. They also provide an enormous opportunity to develop and produce the infrastructure, skills and institutional know-how required to deliver an mRNA therapeutic clinical service. Study sponsors will empower trial sites with the necessary training, resources and equipment to adequately conduct the study, and this model of delivery can provide the blueprint for accepted standards of care.
Despite these numerous and significant benefits, along with the UK’s reputation for being a world leader in clinical trials, there are still several challenges. Industry sponsors have reported a degree of reticence towards running large late-phase studies in the UK, with other countries – particularly the US – seen as much more favourable despite higher reimbursement costs. Trial investigators working in the NHS are facing significant headwinds and trial capacity in UK hospitals is stretched.
There are several quick wins where radical but relatively straightforward policy decisions could substantially shift the dial and put the UK at the forefront of mRNA clinical-trial activity. The following areas are ripe for improvement.
Establishing Trials
Setting up a clinical trial at a study site (an NHS trust for instance) is a lengthy and arduous process that includes a large administrative burden, involvement of numerous departments and multiple site visits. This is particularly acute in clinical trials for mRNA-based therapies that are more complex, with greater demands on services – such as pathology and pharmacy – than other study types.
For each study site, the setup process must be repeated, which can often feel like reinventing the wheel. There is little to no knowledge transfer from neighbouring locations and many NHS trusts are unwilling to accept standard operating procedures (SOPs) – written instructions that describe how to perform a routine or repetitive task in a health-care setting – from external sources. This can put the brakes on site setup and recruitment of participants, and disproportionally affects smaller district centres that may have more personnel constraints and less institutional experience. It is also fundamentally inefficient and a waste of resources to duplicate workload throughout one organisation (the NHS).
The end-to-end setup and recruitment timelines are as important as MHRA approval timelines in getting trials up and running. While the MHRA has improved its approval timelines, this was not matched in other areas such as site setup, costing and contracting, and other interactions. As a result, the end-to-end timelines that drive overall performance and UK competitiveness have not changed significantly, and still lag behind other countries. In the most recent performance reports for the National Institute for Health and Care Research (NIHR) Research Delivery Network, only 26 per cent of studies were open to recruitment within 60 days of their MHRA approval letter.[_]
Supporting knowledge transfer between sites and sharing SOPs, protocols and standards would reduce the workload involved in establishing trial sites for mRNA therapeutics, with NIHR guidance documents being used by trusts as a blueprint for setup. Following a standardised blueprint and sharing knowledge from other trusts with similar circumstances and nuances can also help speed up the process, as demonstrated in the setting up of the Advanced Therapy Treatment Centre network (ATTC) for advanced therapy medicinal products (ATMPs). This collaborative initiative was designed to accelerate the development and delivery of ATMPs, and to advance clinical trials in this area. A similar programme could be set up for mRNA therapeutics, leveraging knowledge from the ATTC network and allowing the UK to continue being a pioneer in this space.
Recommendation: The Department of Health & Social Care (DHSC) should establish a national team, similar to the ATTC network, that can facilitate knowledge transfer around study-site setup for mRNA trials across NHS trusts. This group could work with existing sites to share SOPs, protocols, standards and committee-review outcomes with other NHS sites, and streamline the setup process. NIHR-approved documents and processes should be accepted by all NHS trusts, while patient and public opinions should be sought as to the acceptability of such a process. These should be benchmarked against international best practice.
Recruiting for Trials
The identification of participants for clinical trials remains a relatively manual process. Clinicians are expected to be aware of existing studies that are open for recruitment, retain understanding of inclusion and exclusion criteria, and identify and approach patients on an ad hoc basis during clinical interactions (usually in an outpatient clinic or following a multi-disciplinary team meeting).
This process has several drawbacks. First, it is geographically restricted, with recruiters having limited knowledge of trials outside their area and a relative lack of national matching tools. Second, it is likely to lead to eligible participants not being approached for studies. Third, it means recruitment occurs on a random and often batched basis, usually following a clinic on a particular day of the week. This uncertainty around participants joining trials creates peaks and troughs in workloads for sponsors, research staff, pharmacists and radiology, and adds to the burden of trial delivery; a steady flow of participants is much more desirable. All these issues are particularly pertinent in mRNA therapeutics.
There are several examples of UK leadership in the recruitment space, including the Cancer Vaccine Launchpad (CVLP), credited with driving participant enrolment in a number of mRNA-based cancer-vaccine studies. The associated press releases and case studies for this programme have led to patients voicing positive sentiment towards mRNA cancer vaccines. Further, NHS DigiTrials offers several novel tools that are leveraging national data sets for the design of trials and participant recruitment. These initiatives should be celebrated, supported and replicated as much as possible.
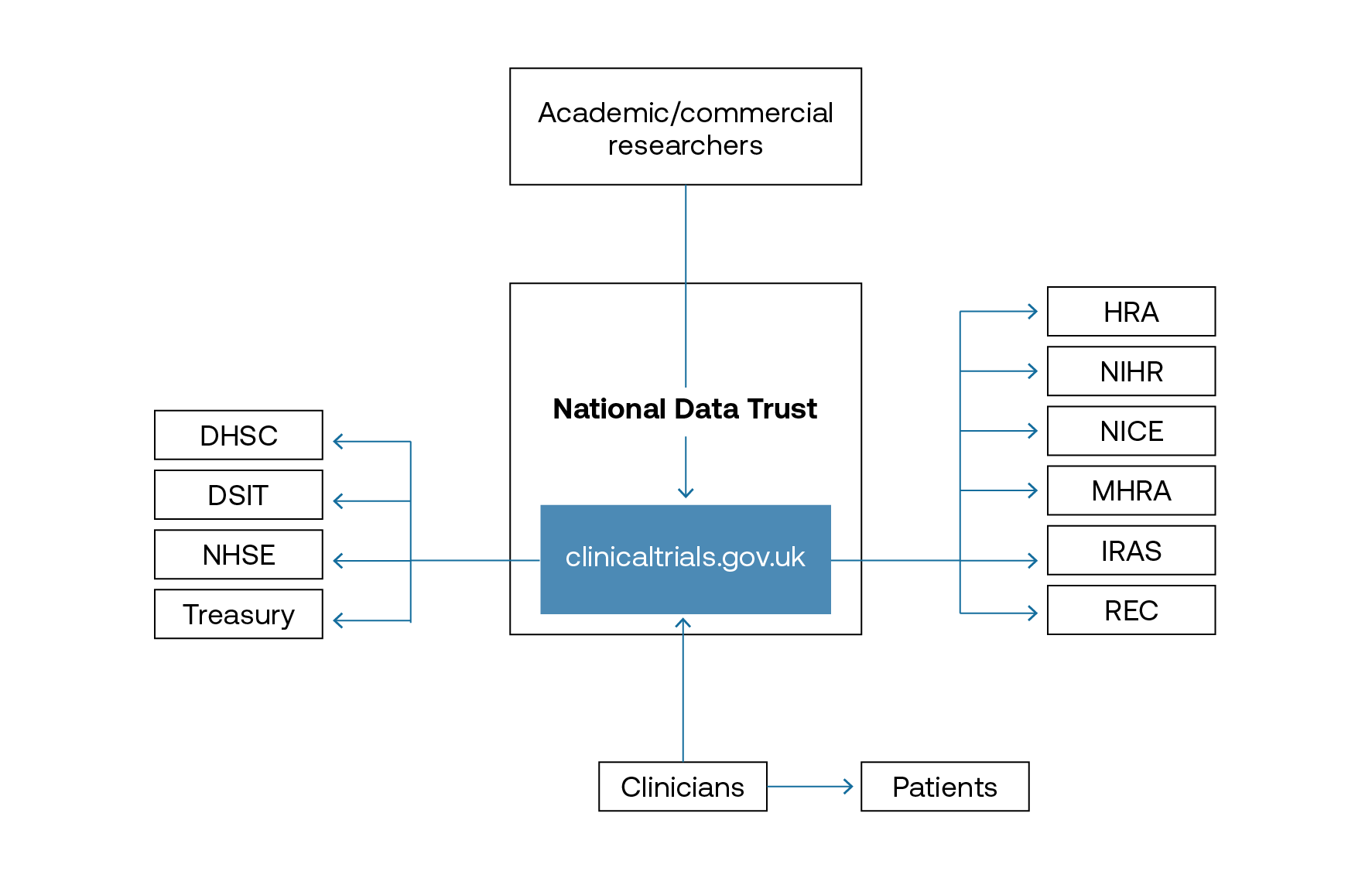
Several matching tools for clinical trials – based on a patient’s electronic health record and a registry of open trials – are being developed by a range of providers. These will be able to better support recruitment but they remain a fragmented system of identification. As suggested in our paper A New National Purpose: Harnessing Data for Health, establishing a National Data Trust with a clinical-trials concierge service would support easier recruitment for trials as a one-stop shop. This service would be enhanced by integrating the O’Shaughnessy review recommendations,[_] such as creating clinicaltrials.gov.uk as a directory of clinical-trial activity in the UK; it would also reap financial benefits for the NHS. Integrating the existing NIHR and DigiTrials infrastructure should mean easier identification of cohorts across the country, enabling better delivery of trials to eligible patients.
Recommendation: Establish clinicaltrials.gov.uk within the National Data Trust to better target patient recruitment for clinical trials, All relevant players, including patients, clinicians and those in industry, would have access.
The National Data Trust will better enable patient recruitment
Resourcing Trials
Clinical trials are a significant source of income for NHS trusts because they are paid to treat, scan and follow up with patients, rather than having to spend money on any of these aspects. However, clinical-trial activity is rarely prioritised. There are very few cases of ringfenced clinician time, specific compensation for trial activity or allocation of trial income to support investment in trial-delivery services or infrastructure.
There is limited understanding of the complexity of mRNA therapeutics and the challenges of delivering this type of clinical trial, with NHS leadership considering all trial activity as equal, regardless of the differing practical aspects. Trial investigators speak of being overwhelmed by bureaucracy, inadequately compensated and excessively burdened by NHS service delivery, creating an environment that stifles the desire to conduct complex studies – such as those involving mRNA.
Not securing time and income for clinicians to conduct clinical trials will have several knock-on effects: patients will not be able to access treatments; the system will not learn how to deliver novel therapeutics; clinicians will not want to deliver trials; industry members won’t have sites to do trials if staff are unwilling; and the NHS will not be able to get much-needed funds reimbursed.
Incentivising clinicians to participate in trials, regardless of their centre size, is central to patients getting access to trials – without this, the UK’s competitiveness as a place to do cutting-edge research declines. Trials should be costed appropriately, with competitive but fair reimbursement rates comparable to international standards. The National Contract Value Review is a good step forward,[_] reducing the setup time for clinical trials with a capacity-building rate built in, but more should be done to direct income to clinical-trial activity than, for example, organisation-wide business-case development.
Recommendation: NHS trusts should ringfence income from clinical trials and allocate this exclusively for clinical-trial activity, including the introduction of protected time (in terms of number of programmed activities for clinical investigators), and offering training and development activities for clinical and non-clinical trial staff. NHS England should mandate this ringfencing and NHS trusts should be obliged to publicly declare their trial income and how this has been reinvested.
Regulating Trials
Since Brexit and the Covid-19 pandemic, the UK regulator has not quite managed to maintain its global reputation; many decision-makers view its processes as too cumbersome, slow and restrictive, with a lack of predictability and capacity in some regulatory procedures. This has become a barrier to opening clinical trials in the UK.
The MHRA acknowledges the scale of the issue and constructively responded to the O’Shaughnessy review of commercial clinical-trial activity in the UK.[_] This set out a bold vision to double trial activity to get back to the pre-pandemic baseline, then to double again by 2027. The MHRA recognised the need to clear a large backlog of licence applications for medicines and, in October 2024, reported that this was 95 per cent complete,[_] with clinical-trial applications meeting 100 per cent of statutory timelines. It is to be commended for openly and publicly publishing its performance against key performance indicators; it also continues to develop schemes such as the Innovative Licensing and Access Pathway,[_] to streamline and expedite the development of key medicines. It is important to celebrate and publicise this work in order to shift perception of the MHRA back to that of a responsive and agile regulator.
Regulation is the cornerstone of mRNA vaccines and therapeutics. During the Covid-19 pandemic, the MHRA garnered a well-justified reputation for being a fast-moving, forward-looking, pragmatic, flexible and effective regulator. With proactivity and a rolling-review process, it was the first in the world to authorise the Pfizer-BioNTech Covid-19 vaccination, ahead of the United States’ Food and Drug Administration (FDA) and European Medicines Agency (EMA), allowing earlier access for UK residents and facilitating large-scale delivery of limited stock.[_],[_] The world, and the pharmaceutical industry in particular, took notice. The UK was seen as being open for business, with a constructive regulator facilitating R&D and market authorisation. This demonstrates the power of regulation and how it can drive an entire sector.
A successful regulator should be aggressive and ambitious in driving down timelines for scientific advice and clinical-trial authorisation around mRNA technologies, aiming to outperform international competitors. With support from the newly announced Regulatory Innovation Office,[_] the MHRA should publish priority disease areas, identifying and providing the necessary resources to fast track their approvals, as well as finding innovative ways of working, including more involvement from the scientific community which would not necessarily require more financial resource.
The assessment process for clinical trials is lengthy and investigators report that it does not always result in accurate or constructive feedback. Leaning on the clinical academic community to provide input into this process – including supporting the review of applications for clinical-trial authorisation – would facilitate improvements, without the need for significant extra resource.
Recommendation: The MHRA, with support from the newly established Regulatory Innovation Office, should publish priority disease areas. This will mean the MHRA building out its existing fast-tracked clinical-trial authorisation processes, targeting vaccines and therapeutics that can meet these demands, as well as drawing on the deep knowledge of the clinical community to identify pain points and addressing these with its in-house expertise.
Prioritising and developing the UK’s sovereign capabilities in mRNA clinical-trial authorisation would continue to make the country attractive to companies wanting to trial their therapeutics. By building out a base in mRNA technologies as a priority, based on the condition that the majority of vaccines in the pipeline are being built on this platform, the UK will be well placed to lead the world in sharing its knowledge on regulating mRNA – and replicating for other platform technologies. This will also contribute to the UK being able to use regulatory diplomacy as a soft-power tool post-Brexit.
For the MHRA to become a world leader in this space, the UK needs to show demonstrable expertise to other regulators. Currently, the MHRA is a “reference” regulator (accepting decisions from others) but not a “reliance” regulator (exporting decisions elsewhere). The MHRA should collaborate with regulators from trusted geographies – such as the EMA and FDA – so as to work in tandem to avoid duplication, particularly around medicine licensing. However, more could be done in this space, particularly with regards to clinical-trial authorisations and approvals of amendments. For global clinical trials, amendments that are accepted internationally by recognised regulators should be reviewed and accepted in the UK with accelerated timelines. Exporting and importing knowledge and expertise in this way would build a fantastic ecosystem, entrenching the idea of regulatory diplomacy; it will also allow the UK to demonstrate the agility it had during the pandemic in a time of business as usual.
Recommendation: When protocol amendments have been approved by recognised international regulators, the MHRA should review and accept them with an accelerated timeline.
Manufacturing
Having mRNA manufacturing capacity in the UK is critical to the mission of delivering these technologies. First, having the capacity to pivot to delivering vaccinations built on mRNA to respond to emerging infectious diseases builds out the country’s national security capacity. Second, having high-value manufacturing capability in mRNA means that the UK can attract international investment in life sciences, capitalising on its existing success from the pandemic.
The UK’s mRNA manufacturing landscape is in a transformative phase, with the Centre for Process Innovation (CPI) in Darlington and eXmoor Pharma in Bristol serving as the country’s only open-access and licensed good manufacturing practice (GMP) facilities, for both ribonucleic acid (RNA) and lipid nanoparticles (LNPs). This facility represents a critical but limited national capability, functioning as a hub for both research and commercial production activities.
The sector is poised for significant expansion, with Moderna’s recent investment commitment to mRNA manufacturing capacities,[_] expected to be operational next year, highlighting a substantial shift in the UK’s manufacturing capacity for Covid-19 and respiratory-disease vaccinations. These planned facilities demonstrate confidence in the UK’s life-sciences sector and are expected to dramatically enhance domestic production capabilities.
A large number of vaccines that use mRNA technology are in the pipeline in Europe
There are significant advantages to the UK considering mRNA as the technology to invest in. Current state-of-the-art manufacturing methods, such as cell-free in vitro transcription (IVT), possess certain advantages because of their ability to control reactions and high yields per unit volume. However, further innovation is needed, in terms of both quality and quantity. The quality of mRNA made via tried-and-tested cell-free manufacturing (which can result in insufficient reduction immunogenicity and lack purity) might not suffice for advanced applications of mRNA for treatment of cancer, rare diseases and genetic disorders.
At the same time, IVT-based mRNA manufacturing requires a complex supply chain of expensive, hard-to-source raw materials, which can increase the cost of mRNA medicines. Therefore novel methods such as decentralised benchtop mRNA printers, as well as cell-based mRNA design and manufacturing, might be needed. Such designs eliminate the need for expensive and complex raw materials, utilising engineered living cells and their machinery to produce mRNA of interest; this also reduces costs and enables better mRNA medicines.
Quality-control procedures are straightforward due to fewer biological variables, while facilities can be designed compactly and flexibly. This means that as a focus for the UK, both cell-free and cell-based technologies will require little physical infrastructure to achieve impactful outputs, with the ability to scale the production up and down.
Despite some existing capacity for mRNA manufacturing, there are constraints in the UK’s manufacturing capabilities. It is possible for mRNA manufacturing facilities to be modular; this provides significant operational benefits, such as allowing for rapid deployment of manufacturing capabilities and facilitating easier technology transfer between sites. Manufacturers can adjust capacity based on demand and the initial capital-investment requirements are lower compared to traditional biological manufacturing plants. Looking at today’s needs, as well as potential future pandemic needs – neither of which the UK’s existing capabilities can appropriately respond to – will require different approaches and strategies.
The UK has a strong R&D base for mRNA and several clinical trials in the pipeline, but not having the requisite manufacturing capacity could deter companies from basing themselves here following clinical trials and put them off delivering therapeutics to patients first. Stronger mRNA manufacturing would bolster the country’s capacity and make mRNA the priority, as well as being a proof of concept to build out capabilities beyond this platform.
Scale Up and Scale Down Manufacturing
The current national capacity for scaling up is relatively limited, with few specialised GMP RNA and LNP manufacturing facilities; having strong scale-up capacity for mRNA technologies is critical for the UK’s preparedness for pandemics. If these constraints are not addressed, upscaling in response to the threat of a pandemic will not be possible. Since mRNA is a beneficial technology to manipulate for infectious diseases, the ability to manufacture large quantities to fill and finish vaccines is critical to UK security.
Comparatively little floorspace is required for mRNA manufacturing, so setting up flexible labs could provide an opportunity for the UK to have this pivot potential. Standardised plug-and-play manufacturing modules could revolutionise technology transfer between sites and allow for a modular, flexible system whereby standardised processes, equipment and protocols can be rapidly deployed and reconfigured. This system would enable quick scale-up/down and product switching while maintaining GMP compliance, as seen at the Cell and Gene Therapy Catapult’s Manufacturing Innovation Centre in Braintree, which has implemented a pharmaceutical quality system: a framework that ensures consistent quality and regulatory compliance that can be demonstrated to regulators.[_]
The potential to scale up manufacturing is critical for pandemic preparedness, but being able to scale it down is equally as pertinent, in terms of the creation of personalised therapeutics and in times of business as usual. The UK is a world leader in delivering clinical trials for novel personalised medicines built on mRNA, such as therapeutic cancer vaccines. Hopefully, if these prove efficacious and cost-effective, they will be recommended for use within the NHS and beyond. However, manufacturing these medicines requires only a small quantity of mRNA, and small-batch manufacturing remains a challenge across the field.
This presents an opportunity for the UK to develop the infrastructure to support small-batch manufacturing. This would facilitate clinical-trial development and delivery in the country but would also support easier, more cost-effective and widespread availability of licensed mRNA therapeutics, particularly within personalised medicine.
Distributed manufacturing is another promising opportunity. This would see quantities of RNA being manufactured across the country, enabling manufacturers to better serve local markets and respond more quickly to changes in demand; they would scale up when necessary but manufacture other therapeutics most of the time for business as usual.[_]
Distributing facilities nationwide would reduce the need for extensive delivery logistics, while working from a unified technology platform would facilitate more streamlined regulation and quality consistency across sites. Comparisons can be drawn with laboratory sciences, where each hospital has its own accredited lab for the testing and analysis of blood samples, with niche rare tests and the ability to process a variable number of samples as demand fluctuates.
This approach could be implemented across the UK through distributed funding, focusing on academic and university hospitals because they are small setups with the ability to pivot between different manufacturing outputs. As a result, the ability to scale up mRNA production during a pandemic, as well as providing personalised medicines during endemic times, would be greatly enhanced. New regulation put before parliament by the MHRA in October 2024 on distributed manufacturing will support this model of manufacturing if passed – and will be the first of its kind in the world.[_]
Recommendation: DSIT should establish “lab hotels” across the UK, with the capability to manufacture mRNA, and scale up and down as needed. Normal use would be for contracted distributed manufacture, as well as for small-batch personalised medicines and local research and trial projects. The relevant technologies would be in place to scale up mRNA manufacturing.
Regulate Manufacturing Processes
The mRNA-manufacturing sector faces significant regulatory bottlenecks when making minor modifications to established platform technologies. While the foundation of these platforms has already undergone rigorous safety and efficacy validation, each modification – even minor agent changes – can trigger delays as a reassessment process is undertaken. This creates unnecessary delays in manufacturing timelines and reduces the ability of production facilities to respond to emerging therapeutic needs.
However, there is a clear opportunity to implement an accelerated assessment pathway for facilities that are using validated platform technologies. When manufacturers propose changes limited to single-agent modifications within an established platform, the fundamental safety and operational parameters of the facility remain unchanged. The MHRA could leverage this consistency to implement a streamlined review process that maintains safety standards while significantly reducing approval timelines.
The key to unlocking this opportunity is restructuring how regulatory bodies assess platform modifications. By creating a specialised fast-track process for changes within the manufacturing process using validated platforms, regulators could focus their scrutiny on specific modifications rather than reassessing an entire dossier. This approach would require criteria for what constitutes a single-agent modification, as well as the creation of dedicated assessment teams with relevant experience and the implementation of timelines for accelerated reviews.
The US’s FDA has already begun developing a Platform Technology Designation Program, which aims to speed up the time for platform drugs to reach patients; it will include a more streamlined assessment of whether the manufacturing of therapeutics using platform technologies can be demonstrably equivalent to the speed of current methods.[_] The UK should look to develop something very similar. Implementation of a streamlined approach would present a number of benefits: it would reduce manufacturing delays while maintaining safety standards; enhance UK competitiveness in mRNA manufacturing; enable the more rapid deployment of therapeutic variations; and reduce the regulatory burden on industry and assessors. By showing agility in this space, the UK will attract life-sciences companies to run clinical trials and manufacture in these facilities, bringing jobs, capital and economic growth.
Recommendation: The MHRA should establish a Platform Technology Modification Protocol to create a specialised assessment pathway for single-agent modifications, setting clear timeline targets for assessment (30 days for standard modifications, for example).
Regulation, Recommendation and Procurement
The UK stands at a critical juncture in deciding its approach to regulating, recommending and procuring these technologies. The MHRA, NHS and National Institute for Health and Care Excellence (NICE) need to assess and adapt their methods to maximise the benefits of this platform. If not, complaints about the time it takes and the suitability of UK institutions to harness home-grown medicine will continue.
MHRA Regulation
There is room for driving even more ambitious timelines for the review of platform technologies. The MHRA has made excellent progress in clearing the backlog of medicine approvals but more could be done to speed up the regulatory process for platform technologies – and mRNA is an ideal platform to trial such an approach. The MHRA could drastically reduce regulatory timescales while maintaining its core function of protecting the public.
Platform regulation should be considered whenever an overall process – drug design, manufacture and delivery – is evaluated and approved, and when any therapeutics derived from this approved platform do not require an entire assessment of all processes, but focus only on safety and efficacy. This can speed up the time it takes to go through the regulatory process, getting benefits to patients quicker.
To get the most out of mRNA as a technology that can be used at speed, it would be beneficial for this platform regulation to include aspects including but not limited to the following.
-
Realising the potential of mRNA as a platform technology: Industry sees a significant opportunity to work closely with MHRA to unlock the full potential of mRNA as a platform technology. By keeping the definition of “platform” under regular review, particularly for personalised therapeutics such as cancer treatments, advancements in quality and stability can be appropriately reflected in regulatory frameworks.
-
Adapting mRNA classification for future guidance: While designating mRNA as an advanced therapy medicinal product (mRNA used in infectious diseases are exempt) may currently facilitate the most efficient regulatory pathway, the MHRA should work with industry to explore how future platform-specific guidance could better align with the evolving science. This would not only enhance regulatory clarity but also bolster confidence in MHRA as a forward-thinking, science-driven regulator.
-
Optimising data use and streamlining processes: By leveraging existing data from mRNA-based products, MHRA could reduce duplication in regulatory timelines. A collaborative effort to develop a “site master” approach, starting with mRNA-based infectious-disease vaccines, could serve as a model for improving efficiency and innovation in future regulatory strategies.
One approach would be to create a stream dedicated to platform technologies within the MHRA, similar to or building on the existing Innovative Licensing and Access Pathway programme but specifically for mRNA. The focus on mRNA should be prioritised as it is the platform with the most potential in clinical trials.
Recommendation: The MHRA should establish a dedicated mRNA team for both clinical trial and market approvals, updating their regulatory science for platform technologies before the first cancer vaccine comes through phase 3 trials. The Regulatory Innovation Office should ensure adequate resources and strategic direction to support this activity.
NICE Recommendations and Guidance
Once certain drugs have been regulated, it is up to NICE to provide guidance on whether the NHS should procure them and if it is cost-effective to do so. Following regulation, companies go to NICE with their licensing, evidence of relative clinical effectiveness to existing therapeutic alternatives and cost-effectiveness, along with their proposed cost; after that, NICE makes its evaluation.
If the UK demonstrates a commitment to mRNA-based therapeutics, life-sciences companies will continue to base their clinical trials in the country, feeling secure that their products will get to market quicker than they would elsewhere. This will benefit patients, who will get access to novel treatments first; this is particularly pertinent due to the potential for mRNA to treat rare diseases, which need to be addressed quickly.
NICE ensures a rigorous process, but there are often complaints that it is too slow to come to a decision. Typically, a review process in the UK takes about nine months if recommendation is given in the first instance; if it requires revision, this can increase to 11 months. In comparison, the US’s Institute for Clinical and Economic Review takes about eight months, Sweden’s Dental and Pharmaceutical Benefits Agency about six months and France’s National Authority for Health about three-and-a-half months. The UK’s timelines have significant cost implications for businesses and can be a blocker to patients accessing medicines in the UK.
The dawn of platform medicine has the potential to spur change in NICE’s evaluation methods. Because the majority of the therapeutic remains the same, with only part of the mRNA payload varying, it is possible to imagine a world in which this is done not on an indication-by-indication basis, but across the platform for multiple similar indications – several tumour subtypes, for example. Of course, it is critical to maintain rigour in the process; however, given that the cost, mechanism, delivery route and level of efficacy are likely to be comparable and predictable across a platform, there are opportunities for substantially increasing the efficacy of the process as the stability and quality of the technology improves.
Recommendation: NICE should review its methods for the recommendation of platform medicines so that not every indication would need renewed guidance but can go through an accelerated process as the technology improves. This should take place in parallel with the MHRA licensing process.
Given that this is about getting novel therapeutics to market and patients quickly, there is something to be said about the efficacy of the French and German models: once products are regulated in these countries they can be used by patients, with their cost-effectiveness evaluated afterwards. France has a programme that temporarily authorises use, with the government providing interim funding so that patients can access the technology. Germany uses a provisional pricing model, whereby the medicine is reimbursed by statutory health insurance while value assessment and pricing negotiations occur.[_]
Another benefit of a rapid evaluation model is that it enables more integration of real-world evidence into the evaluation process, helping assess the drug’s real-world effectiveness, safety profile and optimal use conditions. By facilitating earlier market entry and potential financial returns during the assessment period, these models incentivise pharmaceutical companies to invest in innovative and high-risk therapies, especially in areas of high unmet need, and base themselves within the relevant countries to better support the evaluative process. Most importantly, it gives earlier access to novel therapeutics for patients in circumstances where other treatments might be less effective or more invasive – or where no comparable treatment exists.
Something similar to the French and German models is in place in the UK, in the form of the Early Access to Medicines Scheme (EAMS).[_] This aims to give people with life-threatening or seriously debilitating conditions access to medicine that can meet unmet needs but does not yet have marketing authorisation; however, this can be narrow in scope and a lengthy process.
If the UK was to follow the French and German models, this would encourage companies to either remain or decide to base themselves in the UK, knowing they will gain real-world evidence before going to market. Putting this in place for mRNA therapeutics could be a good starting point, as the platform technology means that there is a fairly standard base cost and it has the largest number of therapeutics coming down the line. This type of model would also indicate what infrastructure might be missing in the rollout, which can then be factored into the cost evaluation.
Recommendation: The government should replace the EAMS for mRNA-based products with advanced-access authorisation, to permit regulated medicines to be more rapidly accessed by patients prior to NICE recommendation and NHS procurement. This would enable better evaluation with more real-world evidence and greater impact.
NHS Procurement
The procurement process for medicines is crucial for patients in terms of accessing treatments, but it also demonstrates a commitment to supporting the efforts of the pharmaceutical industry. If the UK did not procure novel therapeutics, such as those built on mRNA, the industry could lose confidence in the market and start looking elsewhere to sell its products, basing larger parts of its business abroad.
In the current system the NHS has a mandatory three months to procure and offer treatments to patients once it has given guidance. This can be at a price agreed by NICE, although additional negotiations can take place between the NHS and the supplier to obtain a more favourable one. However, this process can be disadvantageous to both the NHS and the supplier: for the former, increased administrative burden and time; for the latter, a potentially costly review and approval processes. This process could be overhauled to create win-wins for all parties, through advanced procurement ahead of licensing.
During the pandemic, through strong leadership from Dame Kate Bingham and the Vaccine Taskforce, the UK made some big bets on the success of vaccine development, in particular focusing on what Bingham called “hairy, scary, sexy” mRNA. The approach was to procure vaccines for a negotiated price prior to their success in efficacy, regulation and recommendation, while supporting the trials and manufacturing capacity. As a result, the UK was the first country in the world to administer a Covid-19 vaccination.
This demonstrates how the UK can have vision and ambition in its collaboration with the private sector, taking some risk for mutual reward. While there can be drawbacks, primarily the risk of wasted money if medicines are not efficacious, there is a significant upside: the UK can obtain medicines ahead of the rest of the world at a much more favourable cost, treating patients earlier and cheaper.
The UK should take this approach with mRNA-based therapeutics immediately, as this is a critical moment in ensuring that the NHS is fit for the future. Of course, there is an important balance between protecting taxpayers’ money and seizing opportunities, but to guarantee UK citizens access to cutting-edge medicines, it is worth the calculated gamble – and this case must be made to the public.
Recommendation: The UK should be the home for making big bets on the efficacy of therapeutics and procuring ahead of licensing. The NHS should have an independent board horizon-scanning and assessing the pipeline, negotiating opportunities with industry players ahead of regulation.
Additionally, with mRNA therapies coming through the pipeline, the UK can lead on making a deal that will be attractive to industry. Reimbursement for personalised mRNA therapies has not yet been achieved in any country, but the UK could create an innovative procurement mechanism. This should incentivise companies to carry out their research in the UK, with an understanding that if more than 20 per cent of patients in clinical trials come from the UK, there will be guaranteed procurement of the drug to all eligible patients. Given that oncology is one of the most promising areas for mRNA therapies, the UK should create an oncology mRNA cancer drugs fund, which would be a bespoke pot for purchasing mRNA cancer therapies.
Recommendation: The NHS should build on the successful models for the Cancer Drugs Fund and Innovative Medicines Fund, creating incentives for companies to run clinical trials in the UK. If it means getting treatments to patients faster, the government should commit to procuring those treatments if they are proven effective.
The Skills Mix
The development of a robust and sustainable mRNA ecosystem in the UK hinges on establishing a well-defined strategy for specialised skills development. This should encompass not only medical and clinical expertise but also a wide range of technical and non-medical roles that are vital to research, development and manufacturing operations.
Clinical Skills
The UK’s clinical research base is essential in enabling the development of mRNA therapeutics and overseeing how they are carried out in clinical trials. The UK has a fantastic asset and advantage in this area, with world-class universities that house very strong science bases, and good links between these institutions and hospitals. However, there is a lack of formal training in clinical development at medical school and in postgraduate medical education, resulting in a lost opportunity to develop the next generation of investigators.
By making hands-on research experience mandatory for students in medicine, life sciences and bioengineering, the UK can encourage a culture of innovation in and engagement with mRNA research. Early exposure to research encourages students to consider careers in academia, biotech or pharmaceutical industries, while developing the skills and mindset needed for future advancements in the field. Medical professionals with research experience are better equipped to collaborate with academic and industrial research teams, creating a workforce that excels not only in clinical practice but also in conducting clinical trials and translational research.
The UK should ensure that all clinical students receive formal training in clinical development, including practical placements in trials units. This would help the students gain experience of what this looks like in practice, while building the UK’s research capacity and promoting research as a career opportunity. Similarly, clinical academic career pathways should continue to be supported as much as possible.
There are a number of challenges in recruitment and retention of these skilled professionals, whose numbers have fallen over time. Addressing the root causes is outside the scope of this paper and is currently the focus of work by the Medical Research Council and others; however, we call on policymakers to give this issue urgent prioritisation.
Recommendation: The General Medical Council, Medical Schools Council and specialty royal colleges should integrate research rotations into all clinical training pathways as a core component of accreditation. Measures should be taken to recruit clinical academics to a career in research and clinical development. A workforce evaluation should be undertaken by NHS professionals to plan for the necessary skills mix to deliver mRNA therapeutics at scale.
Non-Clinical Skills
While clinical research skills are a critical component of the UK’s research base, so too are non-clinical skills. One of the primary challenges facing the UK’s mRNA sector is the lack of clearly defined career pathways for non-clinical professionals who play crucial roles in research projects. These specialists – including bioengineers, data scientists, lab technicians and biomanufacturing specialists – often find themselves without clear career-progression opportunities or chances to engage in cutting-edge research long-term.
This is equally pertinent in manufacturing skills. The CPI’s RNA Training Academy[_] in Darlington, the only significant training provider to support the scaleup and manufacturing aspects of RNA technology across UK industry, has trained more than 700 industrial staff across the UK to date. This is a good start – however, there needs to be more awareness of the facility, alongside a diversity of training countrywide.
The existing biopharmaceuticals workforce needs targeted upskilling, particularly in advanced manufacturing techniques. This could be achieved through apprenticeships, short courses and advanced certifications in mRNA-specific manufacturing processes. Additionally, professionals require training in emerging trends, including modular facilities and continuous manufacturing processes – approaches that can significantly reduce costs and improve efficiency in scaling mRNA production, whether for pandemic preparedness or personalised cancer vaccines.
For the UK to be the go-to place for developing mRNA specialisms and, more generally, expertise in life sciences and biotech, having these skills in-country must be a priority, otherwise it risks losing both business and talent abroad. Creating well-defined career trajectories is essential for attracting and retaining top talent, preventing brain drain to other countries with more structured biotech career-development programmes and higher pay. For life sciences and biotechnology to form a large part of the UK’s industrial strategy and growth agenda, these individuals need to be given the opportunities, resourcing and renumeration to ensure retention and demonstrate their value within the UK.
Moreover, many breakthrough developments in mRNA technology stem from collaboration between scientists, engineers and regulatory specialists. This means that bioinformatics experts, process engineers and quality-assurance professionals need to be accessible for researchers, as a way of bridging the gap between research and scalable production.
Recommendation: DSIT should fund the establishment of an industry-led skills council for non-clinical skills, defining professional standards and career frameworks. This should be alongside the publication of transparent pay boundaries for the top 100 companies, to ensure these skilled workers have clear pathways and a competitive deal.
Recommendation: The National Data Trust should house a central bank of flexible skilled workers, with the agility to be deployed onto projects within both academia and industry where there is demand for these skills. Sitting outside nationally agreed pay bands, these workers would get appropriate renumeration for the value they add to the project, negotiable with the appropriate organisation.
Genomics Skills
Enabling the future of personalised mRNA oncology vaccines will require a large genomics infrastructure to deliver them (including sequencing for both tumour and blood samplesvia circulating tumour DNA). The UK has made great strides investing in genomics, but now needs to connect these investments with providing NHS patients with clinically relevant services for these new therapies. A key part of this is the training of health-care staff, especially surgeons and pathologists, on how to take tumour samples in a way that maximises sequence quality. The UK should set up a dedicated training fund for personalised mRNA oncology genomics for pathologists and surgeons, located in preferred centres of excellence across the UK. This, alongside integrating existing UK companies’ genomics capabilities, such as those provided by Oxford Nanopore Technologies, will cement the country’s capabilities in delivering these technologies.
Recommendation: The UK should create seven mRNA oncology genomics centres of excellence in seven hospital sites around the UK, where these centres would develop best practice and become hubs for wider spokes in their regions.
Regulatory Skills
For the UK to be a world leader in mRNA, the necessary regulatory skills must be in place. While the MHRA has resolved some of its capacity concerns, a noticeable skills gap remains for those doing the assessment. If the MHRA was able to address this, the UK might attract more commercial interest and have mRNA technology contribute to its growth agenda, with medicines reaching patients faster.
Further, it is important to be mindful of the relatively expert and technical nature of mRNA therapeutics, and for the regulatory workforce to be recruited and trained to achieve accurate reviews and decisions in a timely manner. As discussed in the clinical-trials section above, as well as seeking more external resource to support this activity, regulators should consider leveraging expertise from the clinical academic community.
There is a sense among the investigator and industrial community that, on occasion, assessors can lack the deep technical expertise to make the relevant judgement, costing time and money for developers and the regulator. The MHRA has expert advisory groups, but ought to employ the knowledge and expertise of the UK’s talented clinicians to support where there are gaps. This was attempted in 2023, with clinical staff receiving training from NIHR as requested by the MHRA when the backlog was high and it had lost a lot of expert reviewers. These staff are willing and able to donate their time to the MHRA to get approvals across the line, understanding what is at stake if timelines are unnecessarily exaggerated.
Recommendation: The MHRA, in conjunction with NIHR, should train staff in regulatory processes to embed some of the technical skills necessary to get mRNA products over the line. They should consider using the expertise of volunteers within the clinical academic community, and train and retain these assessors.
Delivery and Data
The risk of not having delivery infrastructure, or a strategy to deliver novel medicines, should ring alarm bells within DHSC at this pivotal moment, in that it could result in geographical inequalities in access to treatment across the UK. And if the country doesn’t set itself up to deliver personalised medicines and invest in the future of care, how can it claim to be providing world-class treatment?
The UK lacks the comprehensive delivery infrastructure to make these treatments widely available through the NHS; this includes the logistical infrastructure, data infrastructure and physical infrastructure to deliver personalised medicines at scale. Without addressing these shortcomings, especially the latter, the mission for the UK to be a world leader in mRNA technologies beyond R&D becomes redundant.
The rapid advancement of mRNA therapeutics, particularly in oncology, creates both an opportunity and an imperative for action. Several mRNA-based cancer vaccines are in late-stage clinical trials internationally: the UK is currently running 15 cancer-vaccine trials and there are provisions to run cancer-vaccine trials for 11 more tumour types.[_] Without a clear understanding of the current delivery infrastructure, alongside detailed preparation, the NHS risks not being able to deliver these groundbreaking treatments at scale when they become available.
Starting to plan now would be the most sensible way forward to ensure that the UK remains a competitive market. The UK could learn from Australia’s Department for Industry, Science and Resources, which has published an RNA Blueprint[_] outlining actions that academia, industry and government can take to shore up their future as leaders in this space. Publishing such plans indicates to the world that Australia is committed to attracting business and jobs, as well as making this a key part of its industrial strategy, with investment earmarked for realising this ambition.
Create a Delivery Infrastructure
The benefits of these technologies must come to fruition for the good of patients and the country’s industrial strategy. Without a clear understanding of the current landscape and a clear plan for delivering the potential rewards of 21st-century medicine, the UK will be left behind. Patients will suffer invasive, time-consuming therapies and the country will lose high-stakes companies to countries willing and able to foster these technologies.
The current landscape for the delivery of mRNA therapeutics faces several significant challenges. Infrastructure is fragmented across the UK, lacking the cohesion needed for efficient large-scale deployment. Health-care facilities are operating without standardised protocols for handling and administering these treatments, leading to potential inconsistencies in delivery and outcomes. Workforce-planning challenges are equally significant. Despite the imminent arrival of breakthrough treatments, there is insufficient training and preparation of health-care staff for delivery at scale. And the question remains: who will be required to deliver these treatments – your GP, pharmacist or secondary-care providers?
The NHS, as it stands, is reluctant to procure personalised medicines for all the reasons above. It’s not just the cost of the treatment itself that will be expensive, but also the cost of the requisite delivery infrastructure.
The UK government must take several elements into account when it comes to delivering an mRNA strategy
Failing to address these challenges promptly could have far-reaching consequences for the UK’s health-care system. Without proper infrastructure in place, access to potentially life-saving treatments could be severely limited, denying patients the benefits of these innovative and faster-acting therapies. This will impact not only the health of the nation but also the economy.
Delays in establishing proper infrastructure could result in the UK missing a crucial opportunity to position itself as a global leader in mRNA therapeutic delivery. This would jeopardise the country’s competitive position in biotechnology, and affect future investment and innovation in the sector. To address these delivery challenges, the DHSC, working closely with DSIT, should urgently establish a taskforce to create a clear national strategy, engaging with the realities of current NHS pressures. The DHSC should then move on to the phased roll out of personalised therapeutics – starting with mRNA-based cancer vaccines.
Essentially, if the UK does not actively explore ways to deliver personalised medicine despite the pressures that the NHS faces, it risks falling behind in providing the highest-quality care to patients – where other countries are getting ahead.
Recommendation: DHSC should assemble a taskforce with the chief aim of establishing a clear national strategy for the delivery of mRNA therapeutics at scale within the next five years. This should encompass end-to-end delivery, including data and physical infrastructure as well as workforce plans.
Recommendation: The taskforce should engage with NHS England to establish requirements for the widespread delivery of personalised mRNA therapeutics, particularly in cancer care. It should consider cost and interconnection of current and future personalised therapeutics in the required delivery mechanisms.
Revitalise the Data Ecosystem
One thing underpins the entirety of the mRNA strategic plans outlined above, and that is data. The successful delivery of mRNA therapeutics in the UK health-care system requires a comprehensive transformation of how health-care data are collected, managed and utilised. The UK already possesses incredibly valuable data assets for R&D – including NHS digital infrastructure, Genomics England’s databases, cancer registries and various biobanks – but these systems exist in relative isolation, lacking the integration necessary for effective mRNA therapeutic delivery.
To enable effective mRNA therapeutic pathways, the UK needs to create a seamless data ecosystem that connects research, trials, manufacturing and clinical care. There are multiple elements of such a programme.
-
Patient-level data: These needs to be integrated in real-time across primary- and secondary-care settings, and include standardised recording of genetic and molecular markers.
-
Treatment-response monitoring: Health-care providers need standardised protocols for capturing treatment outcomes, monitoring adverse events in real time and collecting patient-reported outcomes. This needs to be coupled with long-term follow-up data and biomarker monitoring to build a comprehensive understanding of treatment effectiveness.
-
Cost and resource-management data: Health-care systems need to track treatment-delivery costs, resource utilisation and workforce allocation while monitoring the financial impact of new therapies.
Much of this is done by industry members at present, when gaining evidence for regulatory and guidance. But continued data collection by the NHS for understanding the optimal operational model is lacking, as well as impact at a national level once implemented. For example, as noted in the Sudlow Review, the MHRA can rapidly access data from a subset of general practices (about 30 per cent) but not on a national scale.[_]
To achieve this vision, several immediate actions are required. First, the establishment of national data standards for mRNA therapeutic delivery, covering everything from common data elements for patient monitoring to quality metrics for manufacturing. Second, the development of integrated data platforms to connect existing NHS systems with manufacturing facilities and enable real-time monitoring capabilities. Third, implementing robust governance frameworks to manage data protection, access control, ethical use and patient consent. All bodies need access to the relevant data sets to effectively manage their workload and create transparency in the system, from NICE having access to real-world data sets (and, in turn, sharing its timelines) to clinicians being aware of what trials are in the pipeline so that they can be prepared to support patient enrolment.
Longer term, the UK needs to develop advanced analytics capabilities for predictive modelling of patient responses, resource optimisation and outcome prediction. These systems should be integrated with research capabilities to support clinical trials, real-world evidence collection and comparative-effectiveness studies.
The technical, governance and operational challenges in implementing this data infrastructure are substantial and success depends on a coordinated national approach. This should include establishing a joint programme between DHSC and the NHS to set standards and coordinate implementation to meet the challenges above.
The ultimate measure of success will be the system’s ability to support improved patient outcomes, more efficient use of resources, better treatment targeting and enhanced research capabilities. In addition, security and patient confidentiality will need to be maintained – all while keeping costs down and working at speed.
Recommendation: The government should seek to work with the NHS, related bodies and industry to consider the most effective way to capture continuous evidence of the efficacy of new treatments. This data should be used to consider the best models of care and ensure the integration of future platform technologies into their workstreams.
Conclusion
The mRNA moment is here. The question is not whether the UK can participate, but whether it will lead. With strategic investment, coordinated effort and a commitment to innovation, the country has the potential to be a global pioneer. If the UK can convene the ecosystem and get its regulation right, the public will have better medicines and the NHS will be better prepared to deliver for the future. It will also demonstrate to industry players that they can have confidence in the UK as the place to base themselves, supporting the country’s growth agenda.
Acknowledgements
We would like to extend our thanks to the experts who offered their advice and guidance in the development of this report.
-
Arne Blackman, Pfizer
-
Stuart Carroll, Moderna
-
Nicolas Chornet, Moderna
-
Abby Clark, BIA
-
Dr Pippa Corrie, Cambridge University Hospitals NHS Foundation Trust
-
Annette England, BIA
-
Brendan Fish, CPI
-
Dr Elisa Fontana, Sarah Cannon Research Institute
-
Miroslav Gasparek, Sensible Biotechnologies, Numenor Global Insights
-
Vishal Gulati, Recode Ventures, Numenor Global Insights
-
Juliana Haggerty, CPI
-
Arun Harish, CPI
-
Dr Will Ince, Cambridge University Hospital NHS Foundation Trust
-
Nick Johnson, Cell and Gene Therapy Catapult
-
Jasmin Kaur, 1Day Sooner
-
Tony Kerr, Moderna
-
Dr Victoria Kunene, University Hospitals Birmingham NHS Foundation Trust
-
Dr Mark Linch, University College London Hospitals NHS Foundation Trust
-
Dr Nangi Lo, Torbay and South Devon Foundation Trust
-
Katriona Methven, Moderna
-
Dan O’Connor, ABPI
-
Dr Christian Ottensmeier, The Clatterbridge Cancer Centre NHS Foundation Trust
-
Dr Miranda Payne, Oxford University NHS Foundation Trust
-
Dr David Pinato, Imperial College Healthcare NHS Trust
-
Catherine Pollard, Moderna
-
Philip Probert, CPI
-
Malcolm Reid, BioNTech
-
Dr Elizabeth Smyth, Oxford University NHS Foundation Trust
-
Dr Stefan Symeonides, Edinburgh Cancer Centre
-
Dr Fiona Thistlethwaite, The Christie NHS Foundation Trust
-
Martin Turner, BIA
-
William Warr, BioNTech
-
Sam Williams, Moderna

